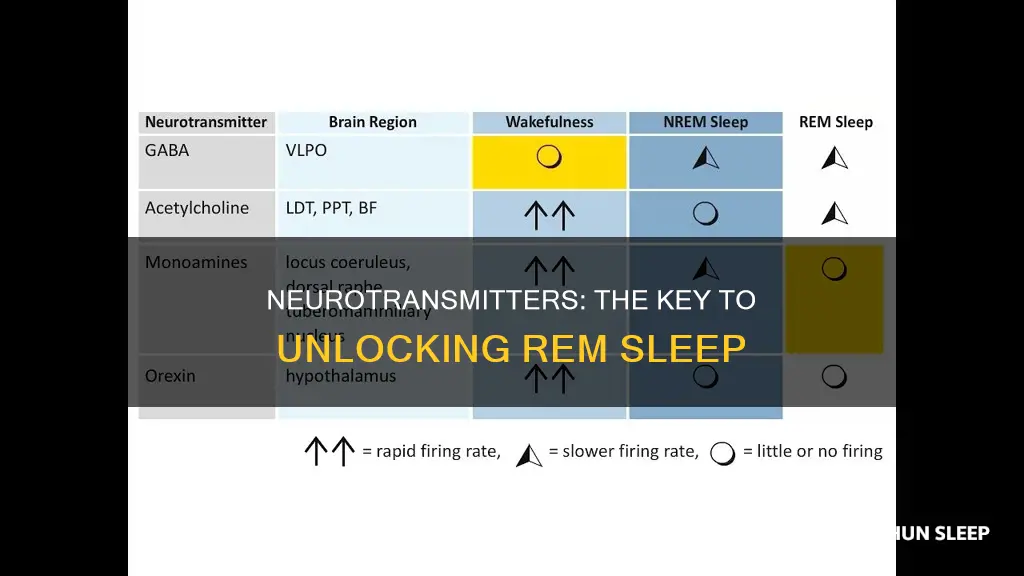
The neurotransmitters that begin and end REM sleep are acetylcholine, GABA, glutamate, glycine, histamine, hypocretin, norepinephrine, serotonin, and dopamine.
REM sleep is generated and maintained by the interaction of a variety of neurotransmitter systems in the brainstem, forebrain, and hypothalamus. The core of the REM-generating circuit is located in the mesopontine junction, medial to the trigeminal motor nucleus and ventral to the locus coeruleus.
The subcoeruleus nucleus (SubC), also called the sublaterodorsal nucleus, is composed of REM-active neurons – cells that are predominantly active during episodes of REM sleep. The majority of REM-active SubC cells are glutamatergic, suggesting that REM sleep is generated by a glutamatergic mechanism. However, GABA SubC cells have also been implicated in REM sleep control.
REM sleep is characterised by rapid eye movements, cortical activation, vivid dreaming, skeletal muscle paralysis (atonia), and muscle twitches. The transition to REM sleep brings marked physical changes, beginning with electrical bursts called ponto-geniculo-occipital waves (PGO waves) originating in the brain stem.
The core brain regions that regulate sleep and wakefulness include the basal forebrain, dorsal raphé nucleus, locus coeruleus, medial preoptic area, prefrontal cortex, and the pontine reticular formation.
| Characteristics | Values |
|---|---|
| Neurotransmitters that begin and end REM sleep | Acetylcholine, Norepinephrine, Serotonin, Histamine, Orexin, GABA, Dopamine, Glutamate, Glycine, Melanin-concentrating hormone, Adenosine |
| Brain regions that regulate REM sleep | Brainstem, Forebrain, Hypothalamus, Pontine Reticular Formation, Dorsal Raphe Nucleus, Basal Forebrain, Ventrolateral Periaqueductal Gray, Dorsal Paragigantocellular Reticular Nucleus, Locus Coeruleus, Medial Preoptic Area, Amygdala, Lateral Hypothalamus, Tuberomammillary Nucleus, Pedunculopontine Tegmental Nucleus, Subcoeruleus Nucleus |
What You'll Learn

The role of acetylcholine in REM sleep
Acetylcholine is a neurotransmitter that is present in both REM sleep and wakefulness. It is one of the few neurotransmitters that are active during REM sleep. Acetylcholine is believed to help the brain keep information gathered while awake and set it while asleep.
Secondly, acetylcholine is involved in the maintenance of REM sleep. Acetylcholine activates cholinergic neurons in the laterodorsal and pedunculopontine tegmentum in the brainstem, which then strengthen transitions into REM sleep.
The cholinergic system, which includes acetylcholine, normally functions to promote REM sleep atonia. However, in patients with REM sleep behaviour disorder, there is a significant degradation of cholinergic centres within the brain.
Setting Up REM Sleep Tracking on Your Fitbit
You may want to see also

The role of the subcoeruleus nucleus in REM sleep
The subcoeruleus nucleus (SubC) is a core region of the brainstem that is active during REM sleep. It is hypothesised that glutamatergic SubC neurons regulate REM sleep and its defining features, such as muscle paralysis and cortical activation.
REM sleep paralysis is initiated when glutamatergic SubC cells activate neurons in the ventral medial medulla, which causes the release of GABA and glycine onto skeletal motoneurons. The activation of the cholinergic REM-active neurons is gated by SubC activity, supporting a mutually excitatory interaction resulting in the generation and maintenance of REM sleep.
The SubC is also implicated in the intrusion of REM sleep paralysis into wakefulness, which is thought to cause cataplexy. Cataplexy is the sudden and involuntary reduction or loss of skeletal muscle tone during otherwise normal wakefulness. Narcolepsy is a sleep disorder characterised by cataplexy and the intrusion of REM sleep into wakefulness.
The subcoeruleus nucleus is also involved in REM sleep behaviour disorder (RBD), which is characterised by the absence of normal muscle paralysis during REM sleep. This is thought to arise from damage to the brainstem circuits that mediate REM sleep atonia.
Understanding Hypnic Jerks: REM Sleep's Mysterious Twitches
You may want to see also

The role of the amygdala in cataplexy
The amygdala is a key node in the brain's emotional regulation circuitry and is believed to play a role in the onset of cataplexy, a symptom of narcolepsy characterised by a sudden loss of muscle tone. Cataplexy is usually triggered by strong emotions such as laughter, anger or surprise.
The amygdala has strong projections to brainstem regions that regulate muscle tone and sleep. It plays a crucial role in the interpretation of emotionally significant stimuli.
Narcolepsy is an incurable sleep disorder characterised by excessive daytime sleepiness, sudden attacks of muscle atonia (cataplexy), fragmented night-time sleep, sleep paralysis and hypnagogic hallucinations. Cataplexy and sleep paralysis are thought to be caused by the abnormal triggering of the REM-sleep muscle tone suppression mechanism during waking.
The amygdala contains a population of sleep-active neurons that increase their firing rate during sleep and cataplexy. These neurons are localised to the central and basal nucleus of the amygdala.
The amygdala also contains a population of wake-active neurons that decrease their firing rate during cataplexy. These neurons are localised to the cortical nucleus of the amygdala.
The anticholinesterase physostigmine, which increases cataplexy, does not alter the activity of cataplexy-related cells, suggesting that its effect on cataplexy is mediated downstream of the amygdala. The alpha-1 blocker prazosin, which also increases cataplexy, increases discharge in a subgroup of cataplexy-active cells, indicating that it may modulate cataplexy by acting on amygdala cells or their afferents.
The amygdala has been shown to make a major contribution to the regulation of emotional processes, particularly to the detection of emotionally significant events and the production of appropriate responses.
REM and NREM Sleep: Understanding Key Differences
You may want to see also

The role of the cholinergic system in REM sleep behaviour disorder
The cholinergic system plays a crucial role in regulating REM sleep, and its dysfunction is implicated in REM sleep behaviour disorder (RBD). RBD is characterised by the absence of normal muscle paralysis during REM sleep, resulting in violent and forceful movements that can lead to self-injury or harm to bed partners.
The core of the REM-generating circuit is located in the mesopontine junction, specifically in the subcoeruleus nucleus (SubC) or sublaterodorsal nucleus. The majority of REM-active neurons in this region are glutamatergic, suggesting that glutamate plays a key role in generating REM sleep. However, GABAergic neurons in the SubC have also been implicated in REM sleep control. Activation of SubC neurons can induce REM sleep and muscle atonia, while lesions in this region can disrupt REM sleep and reduce muscle atonia.
The SubC projects to the ventromedial medulla (VMM), which contains GABAergic and glycinergic neurons that inhibit skeletal motoneurons, leading to muscle paralysis during REM sleep. Both GABA and glycine inhibition of motoneurons are required for REM sleep muscle atonia. Additionally, acetylcholine may also play a role in suppressing respiratory motoneuron activity during REM sleep.
In RBD, there is a breakdown in the communication between the SubC and VMM. Neuroimaging studies have revealed significant degradation of cholinergic centres within the brain in individuals with RBD. This disruption in the REM-generating circuit results in the loss of normal muscle paralysis during REM sleep, leading to the pathological levels of movement observed in RBD.
Furthermore, the cholinergic system in the brainstem, specifically the pedunculopontine tegmentum (PPT), plays a critical role in the regulation of REM sleep. The final activity within the executive cell groups that control REM sleep is determined by the ratio of cholinergic neurotransmission from the PPT to aminergic neurotransmission from the locus coeruleus (LC) and raphe nucleus (RN). Activation of cholinergic cells in the PPT is not only involved in the generation and maintenance of REM sleep but also in the termination of REM sleep episodes by inducing wakefulness.
In summary, the cholinergic system, particularly the SubC and PPT, plays a crucial role in the generation, maintenance, and termination of REM sleep. Dysfunction of this system, specifically the breakdown in communication between the SubC and VMM, contributes to the pathophysiology of RBD by disrupting normal muscle paralysis during REM sleep.
CBD and REM Sleep: What's the Connection?
You may want to see also

The role of the basal forebrain in REM sleep
The basal forebrain is a critical component of the brain's circuitry for regulating sleep. It is composed of neurons that are active during rapid eye movement (REM) sleep, known as REM-active neurons. These neurons produce the neurotransmitter γ-aminobutyric acid (GABA), which plays a crucial role in inducing sleep by inhibiting cells involved in wakefulness. The basal forebrain contains cholinergic neurons that are directly inhibited by GABAergic sleep-active neurons. This inhibition deactivates the cortex, promoting sleep.
The basal forebrain's role in REM sleep is further highlighted by its connection with the hypothalamus, particularly the anterior hypothalamus. The anterior hypothalamus is considered a "sleep center," and lesions in this region can lead to persistent insomnia. The basal forebrain's GABAergic neurons project to the anterior hypothalamus and play a vital role in sleep regulation.
Additionally, the basal forebrain interacts with other brain regions, such as the lateral hypothalamus and the tuberomammillary nucleus, to influence sleep. The lateral hypothalamus contains orexin/hypocretin neurons, which are implicated in narcolepsy and play a role in arousal systems. The tuberomammillary nucleus, which contains histaminergic cells, is involved in the maintenance of wakefulness.
The basal forebrain's complex interactions with various brain regions, including the hypothalamus, contribute to its essential role in regulating REM sleep and overall sleep-wake cycles.
Sleep Stages: The Journey to REM Sleep
You may want to see also
Frequently asked questions
Neurotransmitters involved in REM sleep include acetylcholine, hypocretin, glutamate, and GABA. Acetylcholine is at its strongest during REM sleep and is thought to help the brain keep information gathered while awake. Hypocretin is a neuropeptide that is involved in the regulation of sleep and wakefulness. Glutamate is the main excitatory neurotransmitter in the brain and is involved in the generation of REM sleep. GABA is the major inhibitory neurotransmitter in the brain and is involved in the generation of REM sleep.
Neurotransmitters involved in the transition from wakefulness to sleep include acetylcholine, adenosine, serotonin, norepinephrine, histamine, and dopamine. Acetylcholine is involved in the generation of wakefulness and REM sleep. Adenosine levels increase during periods of prolonged wakefulness and promote sleep. Serotonin, norepinephrine, and histamine levels are highest during wakefulness and promote wakefulness. Dopamine levels are highest during wakefulness and lowest during sleep.
Neurotransmitters involved in the transition from sleep to wakefulness include acetylcholine, hypocretin, and dopamine. Acetylcholine is involved in the generation of wakefulness and REM sleep. Hypocretin levels are greater during wakefulness and REM sleep than during NREM sleep. Dopamine levels are highest during wakefulness and lowest during sleep.